When it comes to fat, it can be a little confusing with regards to which food to choose and which to avoid. For example, vegetable oils like olive oil are 100% fat. It's literally all they contain. On the other hand, a brownie is only around 23% fat. So how come olive oil is healthy and good for you, and the brownie isn't? What's going on?
More...
You may be surprised to learn that the type of fat we eat is more important than the amount. Our weight, blood cholesterol level, and risk of heart disease aren't affected as much by healthy fats as they are by unhealthy fats.
But hold, on a second. Let's start at the beginning.
What is fat in food?
You may have heard that oily fish is good for you and should be eaten regularly as part of a healthy diet.
(If you haven't, well then I guess you have some catching up to do on my blog!)
Under a microscope, if we were to focus on an individual globule of fat we'd see something interesting.
We'd see that what we think of as fat is actually made up of other individual molecules joined together
We call these molecules triglyceride.
Triglycerides come in many shapes and forms and have different properties depending on their structure.
Here's an example *cue flashback to your high school chemistry days...*
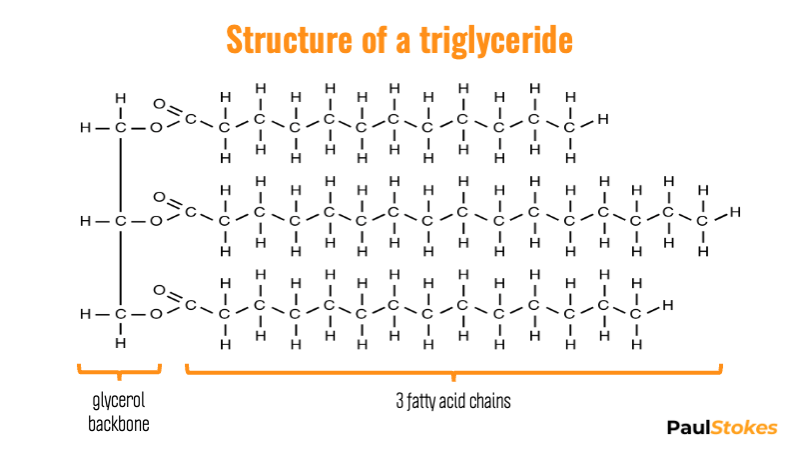
Adapted from OpenStax College, CC BY 3.0
You'll see 3 carbon atoms up the left-hand side. That's the glycerol backbone.
Essentially, glycerol holds the rest of the molecule together.
You'll see three long chains on the right, attached at one end to the glycerol carbons. These chains are called fatty acids.
The structure of the fatty acids basically determines everything about them.
For example, whether they are liquid or solid at room temperature. Think of the difference between butter and olive oil.
The structure of the fatty acid also decides how quickly that oil will go rancid.
And perhaps the most crucial aspect, the structure determines whether that fat is good or bad for your body.
Some simple differences between fatty acids have BIG effects on the food
The first aspect we'll look at is length.
Some fatty acid changes can be short, others are long.
A perhaps more important difference is how the carbon atoms are bonded together in the chains. Some fatty acids will only have single bonds while others will have a mixture of single and double bonds.
The difference in foods with saturated versus unsaturated fats
Saturated fatty acids that only have single bonds are what we call saturated. Their molecules are literally 'saturated' with hydrogen - they can't absorb any more into their structure.
Fatty acids that have one or more double bonds between carbon atoms are known as unsaturated.
Generally speaking, too much saturated fat is bad for you. On the other hand, unsaturated fats are good for your body.
How do I know which fats are saturated and which are unsaturated?
Well, an easy way to tell is that saturated fats are generally solid at room temperature. Unsaturated fats tend to be liquid, like oils.
Why?
Well when you look at the structure, saturated fats' bonds are all single and regular. The molecules are uniform, with no kinks or bends in their chains. As a result, they can stack neatly together to form a 'solid' structure.
Our body doesn't care how a molecule looks on paper. It only cares about where that molecule fits, what it reacts with, and how it can use it.
Unsaturated fats on the other hand, with their double bonds, have chains of different shapes. This means they don't stack so neatly together at a molecular level. Consequently, it takes more energy to get them to a solid-state hence why they remain liquid at room temperature.
What about the different types of unsaturated fat in my food?
For saturated fats, the above is all you pretty much need to know.
With unsaturated fats, things get a little more complicated.
Due to the way the double bonds work, there are essentially two different ways to arrange each molecule.
Cis and Trans fatty acids
When the molecule forms with both hydrogens on the same side, and both carbons on the opposite side. This is known as the cis configuration.
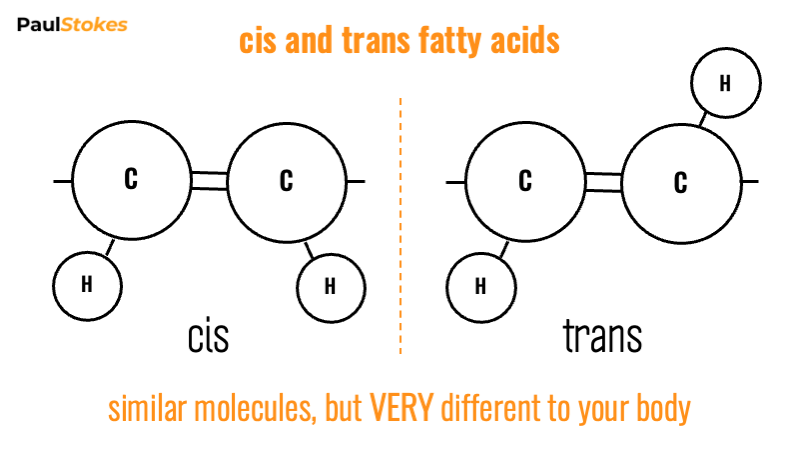
However, when the fatty acid molecule forms with the pairs of hydrogens and carbons on opposite sides of the bond, we get the trans configuration.
Now, you may be thinking "What's the big deal?"
While both molecules contain the same atoms, in the same order, they have one important difference.
The overall molecules will have a different shape from one another.
And that has a HUGE impact on your body. The molecules will behave differently as our enzymes, organs, and cells try to process them
Our body doesn't care how a molecule looks on paper. It only cares about where that molecule fits, what it reacts with, and how it can use it.
You've probably heard of trans fats before. They don't go rancid, they're more stable during deep frying and they can change the texture of foods in ways that other fats just can't.
Trans fats are BAD for your health
Trans fats came about in food production since they don't go rancid and can modify the texture of our food. They have properties that other fats don't.
Even though technically, trans fats are a type of unsaturated fat, they're bad for us.
Worse even than saturated fats.
Trans fats only exist in very small quantities in nature. Our body can't process them, or transport them, in the same way, it can with other fats.
How do I know which food has trans fat?
Well, it can sometimes be a little tricky.
In Australia and other parts of the world, there is no legal requirement to show trans fats on nutrition labels.
A survey by Food Standards Australia New Zealand in 2009 found that levels of trans fat have been decreasing in our food. We generally fall within the guidelines the World Health Organisation recommends. They felt that the population is generally within the accepted safe limits for consumption.
However, if you are concerned, it always pays to read the packaging thoroughly. If you read the ingredients list and you see 'partially hydrogenated' you can be sure that the product contains some trans fat. Basically, that's how industry makes trans fats in food production - by adding hydrogen, or 'hydrogenating' unsaturated fats.
What Food Standards Australia New Zealand did advise though, is that Australians would do well to reduce their overall consumption of saturated fats.
So how does all this fat knowledge apply when making healthy food choices?
Well, let's revisit our olive oil vs brownie debate from earlier.
Olive oil is 100% fat whereas a brownie is only 23% fat. However, olive oil is mostly unsaturated fat. Furthermore, it has no trans fat at all.
On the other hand, saturated fat makes up more than half of the fat in a brownie. So, even though olive oil has more fat, it's healthy. The brownie isn't.
Remember, it's the type of fat that's more important for our health than the amount.
And I'm not here to bash brownies. Everything is fine in moderation.
Furthermore, there are lots of food choices that have equally bad, and worse, fat profiles.
Just remember to consider the type, and ultimately the shape of the fat. That's what your body does.

READ ALSO: Good fats vs bad fats
